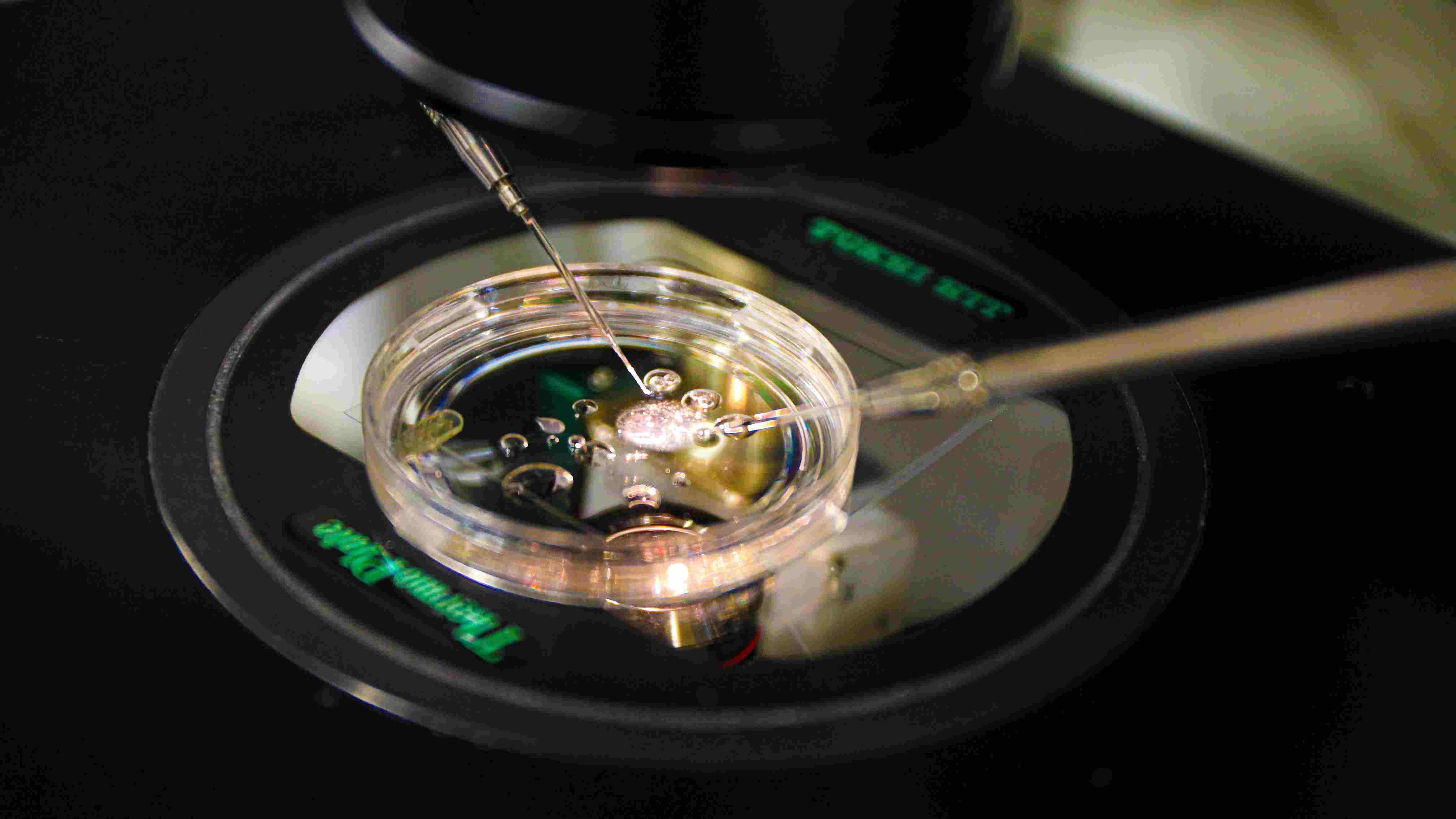
A pioneering experiment by US scientists to create embryos from skin cells has opened up new possibilities for fertility treatment and parenthood, including giving same-sex couples the chance to have children genetically related to both partners.
The research, led by Oregon Health and Science University (OHSU), marks the first time early-stage human embryos have been produced using DNA from skin cells and fertilised with sperm.
The innovation focuses on overcoming infertility caused by age, illness, or lack of viable reproductive cells. It uses the nucleus from a skin cell—which holds all the genetic information needed to build a body—and transfers it into a donor egg that has been emptied of its DNA.
This step mirrors the cloning process used to produce Dolly the Sheep in 1996, but with a crucial difference: the egg must then discard half its chromosomes to prepare for fertilisation.
To make this possible, researchers developed a special cell division process called “mitomeiosis”, combining elements of mitosis and meiosis. Through this, the egg is prompted to remove half of its chromosomes, leaving the right amount to merge with sperm.
Using this method, scientists generated 82 functional eggs, fertilised them, and observed some developing into early-stage embryos. None were grown beyond six days, but researchers say the experiment proved a key concept many believed was unattainable.
“We achieved something that was thought to be impossible,” said Prof Shoukhrat Mitalipov, the director of OHSU’s Centre for Embryonic Cell and Gene Therapy.
Although promising, the process is still in its early stages. The egg discards chromosomes randomly, which can lead to errors, and the success rate so far is low at around 9 percent.
The chromosomes also skip a natural rearrangement process known as crossing over, which is important for healthy development.
Prof Mitalipov said improving accuracy will be the next step. “We have to perfect it. Eventually, I think that's where the future will go because there are more and more patients that cannot have children,” he said.
This discovery is part of a fast-evolving scientific field called in vitro gametogenesis, which aims to create eggs and sperm outside the human body.
If perfected, it could benefit a wide range of people: older women with no viable eggs, men with low sperm counts, cancer survivors whose fertility was affected by treatment, and same-sex couples who want children carrying both their genes.
Prof Paula Amato from OHSU said: “In addition to offering hope for millions of people with infertility due to lack of eggs or sperm, this method would allow for the possibility of same-sex couples to have a child genetically related to both partners.”
Experts say the breakthrough raises important ethical questions and calls for strong public engagement.
Roger Sturmey, a professor of reproductive medicine at the University of Hull, said the study highlights the need for clear communication and strong oversight.
“At the same time, such research reinforces the importance of continued open dialogue with the public about new advances in reproductive research. Breakthroughs such as this impress upon us the need for robust governance, to ensure accountability and build public trust,” he said.
Prof Richard Anderson, deputy director of the MRC Centre for Reproductive Health at the University of Edinburgh, added that the ability to generate new eggs “would be a major advance,” though safety concerns must be addressed before moving forward.
“There will be very important safety concerns but this study is a step towards helping many women have their own genetic children,” he said.
While it may take up to a decade before fertility clinics can consider using the method, scientists say the experiment has changed what is possible in reproductive medicine, laying the groundwork for future families to be created in entirely new ways.